When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. 2 HCl aq Ba OH2 aq BaCl2 aq 2 H2O l ΔH -118 kJ Calculate the heat when 1148 mL of 0500 M HCl is mixed with 3000 mL of 0400 M Ba OH2.
Solved 16 Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com
Hydrochloric acid reacts with barium hydroxide according to the equation.
. Consider the following reaction. 2HClaqBaOH2AQ-BaCl2aq2H2Ol DeltaH -118 kj Calculate the heat in kj associated with the complete reaction of 365 grams of HCl aq. Hydrochloric acid reacts with barium hydroxide according to the equation.
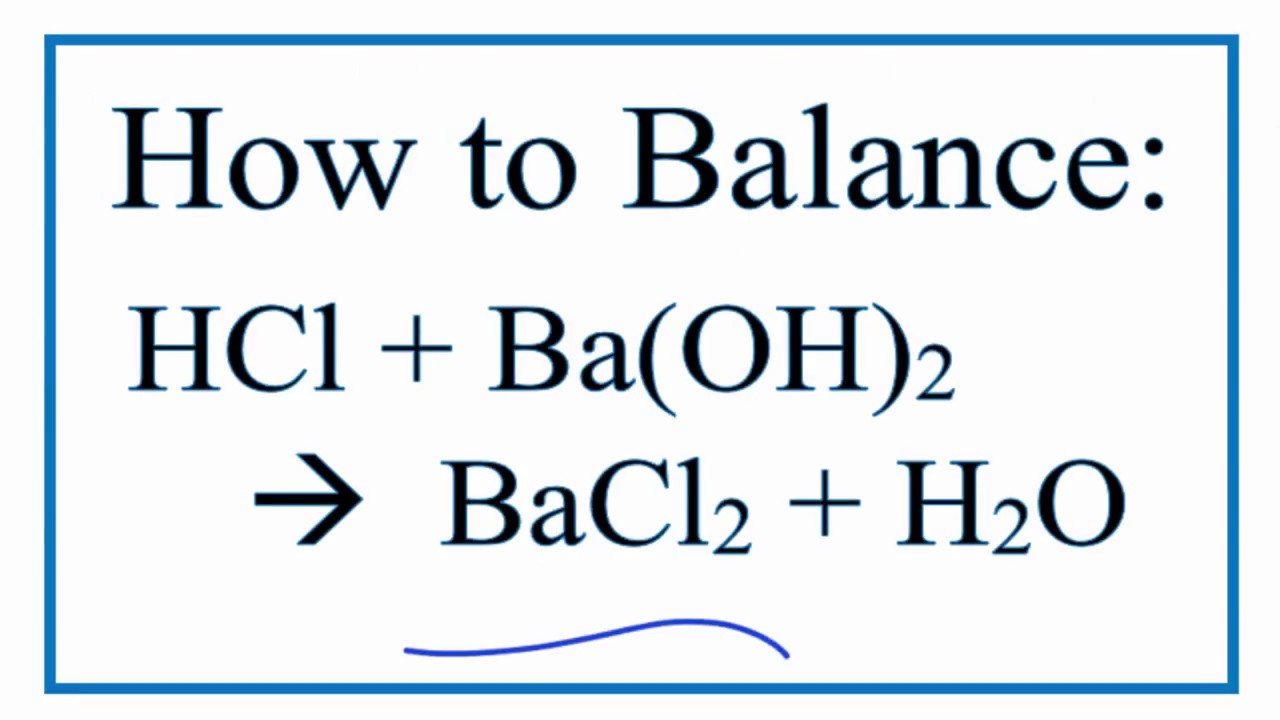
In this video well balance the HCl BaOH2 BaCl2 H2O and provide the correct coefficients for each compound. 2 HCl aq Ba OH2 aq BaCl2 aq 2 H2O l ΔH -118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water.
The equation for this reaction is Ba OH2 2 HCl BaCl2 2 H2O. Hydrochloric acid reacts with barium hydroxide according to the equation. E none of the above.
B a 2 H C l B a C l 2 H 2 When barium reacts with hydrochloric acid it will form barium chloride with the evolution of hydrogen gas. Ba OH2 is a strong base while HCl is a strong acid. When hydrochloric acid reacts with barium hydroxide barium chloride and also water room produced.
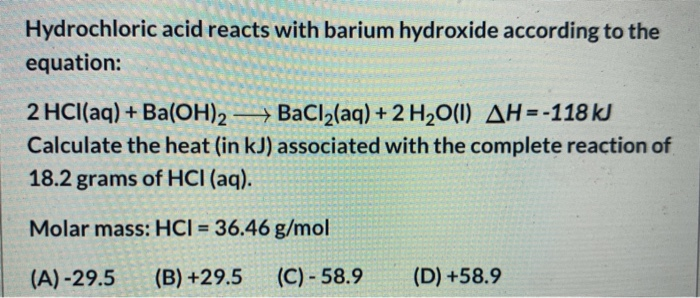
Hydrochloric acid reacts with barium hydroxide according to the equation. 2 HCl aq BaOH2 aq BaCl2 aq 2 H2O l Δ H -118 kK Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. The balanced equation because that this reaction is.
Here 1 mole of barium reacts with 2 moles of hydrochloric acid to produce 1 mole of barium chloride and 1 mole of hydrogen gas. BaCl2 2H2O What is the equation of barium sulphate with hydrochloric acid. What is the balanced equation for the reaction of hydrochloric acid and barium hydroxide.
468-100 mL X343 mL X 16 g M of BaOH2 16 1713 934x10-3mol 934x10-3mol 00343 027 M BaOH2 2HCl BaCl2 2H2O according to the ballanced equation 1 mol base neut. 2 mol acid VM mol. In a balanced equation the number of atoms of each element will remain equal.
2 HCl aq Ba OH2 aq BaCl2 aq 2 H20 1 Hrxn -118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. Hydrochloric acid and barium hydroxide. 2HCl BaOH2.
Now barium hydroxide BaOH_2 will react with hydrochloric acid HCl to produce aqueous barium chloride BaCl_2 and water according to the balanced chemical equation BaOH_ 2aq colorred2HCl_ aq - BaCl_ 2aq colorblue2H_ 2O_l Notice that you have a 1colorred2 mole ratio between barium hydroxide and. 2 HCl aq Ba OH2 - BaCl2 aq 2 H2O 1 AH-118 kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. Zinc metal reacts with hydrochloric acid according to the following balanced equation.
Hydrochloric acid reacts with barium hydroxide according to the equation. Assuming that the temperature of both solutions was initially 250C and. Calculate the heat in kJ associated with the complete reaction of 182 grams of HCI aq.
2HClaqBaOH2AQ-BaCl2aq2H2Ol DeltaH -118 kj Calculate the heat in kj associated with the complete reaction of 365 grams of HCl aq. 2 HCl aq BaOH2 aq BaCl2 aq 1H2O l H -118 kj Calculate the heat in kJ associated with the complete reaction of 182 grams of HCl aq. Barium hydroxide react with nitric acid Ba OH 2 2HNO 3 Ba NO 3 2 2H 2 O Check the balance Barium hydroxide react with nitric acid to produce barium nitrate and water.
30 Hydrochloric acid reacts with barium hydroxide according to the equation. HCI 3646 gmol A-295 B 295 C -. BaCl2 aq 2 H2O l H -118 kJCalculate the heat in kJ associated with the complete reaction of 500 mL of 0250 M HCl aqA.
Hydrochloric acid reacts with barium hydroxide according to the equation. At 25C solubility of BaOH2 468 g in 100 mL so we have. 2HCl aq Ba OH 2 aq â BaCl2 aq 2H2O l If 4 mole of barium hydroxide react the reaction consumes ____ mole of hydrochloric acid.
Hydrochloric acid reacts with barium hydroxide according to the equation2 HCl aq Ba OH2 aq. 16 Hydrochloric acid reacts with barium hydroxide according to the equation. Learn how to use the molecular equation to write the complete ionic and net ionic equations for a reaction occurring in aqueous solution.
Chemistry questions and answers. Hydrochloric acid reacts with barium hydroxide according to the equation. Magnesium metal reacts with hydrochloric acid to produce hydrogen gas H2 Mg 2HCl ---- MgCl H2 Calculate the volume in liters of hydrogen produced at 33 degrees C and 665 mmHg from 00840 mol Mg and excess HCl P 875 atm V Chemistry.
To balance HCl BaOH2 BaCl2 H2O. HClaqBaOH2aq ----- BaCl2aqH2Ol and also given Delta H -118kJ Calculate the heat in kJ associated with the complete reaction of 182 grams of HClaq. 2HClaq BaOH2 aq BaCl2aq 2H2 01 If 4 mole of barium hydroxide react The reaction consumes moles of hydrochloric acid.
The balanced equation for this reaction is.
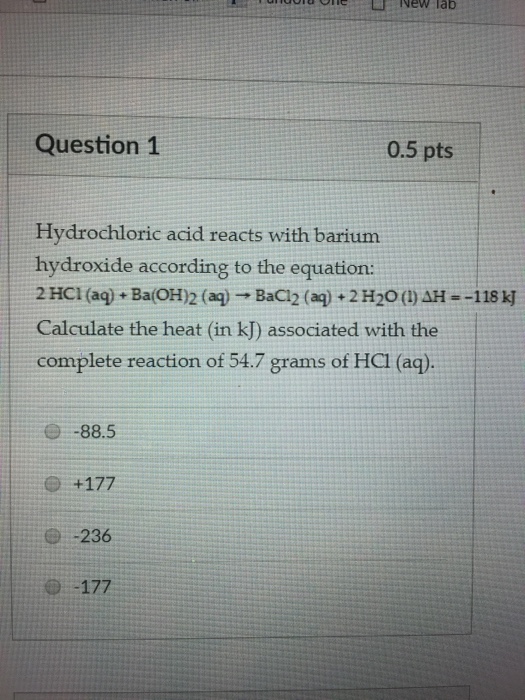
Solved New Lab Question 1 0 5 Pts Hydrochloric Acid Reacts Chegg Com
Solved Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com

How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube
0 Comments